Henlius Unveils Updated Phase 2 Results and Phase 3 Trial Design for Anti-HER2 mAb HLX22 in First-Line HER2+ Gastric Cancer
On June 2, 2025, Henlius (2696.HK) announced that the latest research results of novel anti-HER2 monoclonal antibody (mAb) HLX22 were presented at the 2025 American Society of Clinical Oncology (ASCO) Annual Meeting. These included updated efficacy and safety data with over two years of follow-up from the phase 2 clinical study (HLX22-GC-201) evaluating HLX22 in combination with trastuzumab and chemotherapy as a first-line treatment for HER2-positive advanced gastric cancer, as well as the debut of the study design for the international multicentre phase 3 clinical trial (HLX22-GC-301), which features a head-to-head comparison with the first-line standard of care therapy (trastuzumab + chemotherapy ± pembrolizumab).

Updated data from the HLX22-GC-201 study demonstrated that the efficacy benefit of HLX22 in HER2-positive gastric cancer remained stable with extended follow-up, outperforming previous data. Meanwhile, the HLX22-GC-301 study is progressing efficiently worldwide and has already completed its first patient dosing in multiple countries and regions. As of now, no similar dual HER2 blockade therapy for the treatment of HER2-positive gastric cancer has received approval for commercialization globally.
Dual-Blockade, Dual-Epitope HER2 Therapy Breaks Through First-Line Treatment Barriers in Gastric Cancer
Until now, gastric cancer still constitutes a major global health problem. According to GLOBOCAN 2022, there were around 1 million new cases and over 660 thousand new deaths of gastric cancer in 2022 globally [1]. Gastric cancer is often diagnosed at an advanced stage, with a poor prognosis and a 5-year relative survival rate of only 6%[2,3]. Despite the advancements in targeted therapies, such as anti-HER2 agents, and immune checkpoint inhibitors (anti-PD-1/PD-L1 mAbs) for gastric cancer treatment in recent years[4], the disease's high molecular heterogeneity leads to markedly varied responses to chemotherapy, targeted therapy, and immunotherapy across different subtypes[5]. Immunotherapy remains limited to PD-L1 positive populations with only modest efficacy improvements. This underscores the urgent unmet clinical needs in the overall management of HER2 positive gastric cancer.
HLX22, an innovative anti-HER2 mAb, was granted Orphan Drug Designation (ODD) from both the FDA and the EC for the treatment of gastric cancer. HLX22 can bind to HER2 extracellular subdomain IV at a binding site different from that of trastuzumab via differentiated molecular design and mechanism of action, which allows simultaneous binding of HLX22 and trastuzumab to HER2 dimers (HER2 homodimer and HER2/EGFR heterodimer) on tumour cell surface, resulting in a 40%–80% increase in HER2 internalisation. thereby strengthening the blockade of HER2-mediated signalling pathways. Pre-clinical studies showed that the co-treatment with HLX22 and trastuzumab synergistically inhibited tumour cell proliferation and apoptosis, which led to enhanced anti-tumour activity in vitro and in vivo.
Phase 2 Data Highlights: Significantly Prolonged PFS & OS with Over Two Years of Follow-Up
The phase 2 clinical data on the combination of HLX22 and HANQUYOU (trastuzumab, trade name: HERCESSI™ in the U.S., Zercepac® in Europe) demonstrate that the addition of HLX22 to trastuzumab plus chemotherapy significantly improves survival and anti-tumour efficacy in first-line treatment of HER2-positive gastric/gastroesophageal junction cancer (GC/GEJC) patients, with manageable safety profiles. This combination holds potential to redefine the first-line standard of care for advanced gastric cancer.
The study data were first presented at the 2024 American Society of Clinical Oncology Gastrointestinal Cancers Symposium (ASCO GI). Subsequent updates were also showcased at prominent journal and conferences, including Med, the 2024 ESMO Gastrointestinal Cancers Congress (ESMO GI), and the 2025 ASCO GI -3. These repeated recognitions not only further solidified the reliability of the findings but also significantly enhanced the study’s influence within the research community.
Patients with locally advanced or metastatic HER2+ G/GEJC and no prior systemic antitumor therapy were enrolled in HLX22-GC-201 and randomized in a 1: 1 ratio to receive either HLX22 + trastuzumab + XELOX or placebo + trastuzumab + XELOX in 3-week cycles. Primary endpoints were independent radiology review committee (IRRC)-assessed progression-free survival (PFS) and objective response rate (ORR) per Response Evaluation Criteria in Solid Tumors (RECIST) v1.1. Secondary endpoints included other efficacy and safety endpoints.
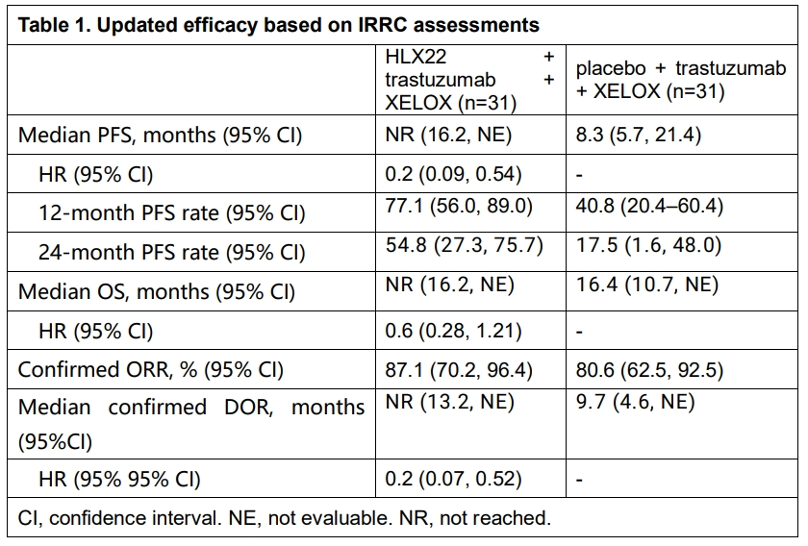
As of December 6, 2024, 62 patients were randomized to the respective groups (31 vs 31), of whom 51 (82.3%) were male. Median follow-up duration was 28.5 and 28.7 months for the respective groups. Major efficacy findings are shown in Table 1. Treatment-emergent adverse events (TEAEs) were reported in 30 (96.8%) and 31 (100%) patients, respectively. TEAEs of grade 3 or higher were observed in 17 (54.8%) and 15 (48.4%) patients. HLX22- or placebo-related TEAE leading to death occurred in 1 (3.2%) patient in the placebo + trastuzumab + XELOX group. One patient (3.2%) in each group reported HLX22/placebo-related TEAEs leading to treatment discontinuation. The updated results of HLX22-GC-201 again demonstrated that combination of chemotherapy and dual HER2 blockade with HLX22 and trastuzumab conferred survival benefit to HER2-positive G/GEJC patients along with a manageable safety profile.
Phase 3 Head-to-Head Trial Design: Global Acceleration of MRCT
Based on the prospective HLX22-GC-201 study, the company further evaluated the efficacy and safety of HLX22 in combination with trastuzumab and chemotherapy as a first-line treatment for HER2-positive advanced gastric cancer across broader populations. A head-to-head international multicentre phase 3 clinical trial (HLX22-GC-301) was subsequently initiated.
Co-led by Prof. Lin Shen from Peking University Cancer Hospital and Prof. Jaffer A. Ajani (MD Anderson Cancer Center; Chair of the NCCN Guidelines Panel for Gastric and Esophageal Cancers) as global principal investigators, the trial design of HLX22-GC-301 was unveiled for the first time at the 2025 ASCO. This randomized, double-blinded, two-arm international phase 3 clinical study aims to compare the efficacy and safety of HLX22 in combination with trastuzumab and XELOX versus trastuzumab and XELOX with or without (±) pembrolizumab in patients with HER2-positive, advanced G/GEJ cancer and no prior antitumor therapy in the advanced setting.
The study’s key inclusion criteria include histologically or cytologically confirmed diagnosis of previously untreated, locally advanced unresectable or metastatic HER2-positive G/GEJ adenocarcinoma. Key exclusion criteria include prior use of any HER2-target therapy. Approximately 550 eligible patients will be enrolled from multiple regions across the globe and randomly assigned in a 1:1 ratio to receive HLX22 (15 mg/kg) + trastuzumab + XELOX ± placebo (for pembrolizumab) or placebo (for HLX22) + trastuzumab + XELOX ± pembrolizumab. HLX22 will be administered intravenously on Day 1 of each 21-day treatment cycle until loss of clinical benefit, death, intolerable toxicity, withdrawal of informed consent, or other reasons. The stratification factors include HER2 immunohistochemistry (3+ vs 2+), geographic region (Asia vs Europe/North America vs rest of the world), primary tumour site (gastric vs gastroesophageal junction), and tumour PD-L1 expression (CPS < 1 or not evaluable vs 1 ≤ CPS < 10 vs CPS ≥ 10). The dual primary endpoints are PFS assessed by independent radiology review committee per RECIST v1.1 and overall survival. Secondary endpoints include investigator-assessed PFS, objective response rate, PFS on the subsequent line of therapy, duration of response, safety, pharmacokinetics, immunogenicity, and quality of life.
Notably, HLX22-GC-301 has set up trial sites across China, Australia, the European Union, Japan, the United States, South America, and other countries and regions. As of now, the first patient was dosed in China, Japan, and Australia, and the study has obtained investigational new drug (IND) approvals in countries and regions including the United States (U.S.), Chile and Korea.
Following its focus on HER2-positive gastric cancer, HLX22’s therapeutic scope has expanded into breast cancer. In April 2025, a phase 2 clinical trial (HLX22-BC201) of HLX22 in combination with trastuzumab deruxtecan (T-DXd) has completed the first patient dosing for the treatment of HER2-low, hormone receptor (HR)-positive locally advanced or metastatic breast cancer in China. Preclinical animal studies of HLX22 combined with T-DXd showed synergistic anti-tumour effect and good safety profiles, which may offer more benefits to patients with HER2-expressing tumours.
Moving forward, Henlius will continue to explore the therapeutic potential of novel HER2-targeted therapies in tumours, accelerating HLX22’s global development to deliver more affordable and effective treatment options for patients worldwide.
Reference:
[1] Bray F, Laversanne M, Sung H, et al. CA Cancer J Clin. 2024: 1-35.
[2] Ajani JA. et al. J Natl Compr Canc Netw 2022;20(2):167-92.
[3] Alsina M. et al. Nat Rev Gastroenterol Hepatol 2023;20(3):155-70.
[4] Miao, ZF.,et al. Progress and remaining challenges in comprehensive gastric cancer treatment. Holist Integ Oncol 1, 4 (2022).
[5] Guan, WL.,et al. Gastric cancer treatment: recent progress and future perspectives. J Hematol Oncol 16, 57 (2023).
[6] Jin Li et al., HLX22 plus HLX02 and XELOX for first-line treatment of HER2-positive locally advanced or metastatic gastric/gastroesophageal junction cancer: A randomized, double-blind, multicenter phase 2 study.. JCO 42, 354-354(2024).DOI:10.1200/JCO.2024.42.3_suppl.354
[7] J. Li et al., 422P HLX22 plus HLX02 and XELOX as first-line therapy for HER2-positive advanced gastric/gastroesophageal junction cancer: Updated results from a randomized, double-blind phase II study, Annals of Oncology,Annals of Oncology (2024) 35 (suppl_1): S162-S204. 10.1016/annonc/annonc1482
[8] Li N, et al. A randomized phase 2 study of HLX22 plus trastuzumab biosimilar HLX02 and XELOX as first-line therapy for HER2-positive advanced gastric cancer. Med. 2024;5(10):1255-1265.e2.
[9] Jin Li et al. HLX22 plus trastuzumab and XELOX for first-line treatment of HER2-positive locally advanced or metastatic gastric/gastroesophageal junction cancer (G/GEJC): Updated results with additional patients.. JCO 43, 440-440(2025). DOI:10.1200/JCO.2025.43.4_suppl.440

