Fosun Pharma's Independently Developed Innovative Lung Cancer Drug Foritinib (SAF-189) Marketing Authorization Application Has Been Accepted by NMPA
March 10, 2025 – Recently,Fosun Pharma announced that the marketing authorization application for its independently developed new drug, foritinib Succinate Capsules (project code: SAF-189, foritinib, hereinafter referred to as "New Drug"), has been accepted by the National Medical Products Administration (NMPA). The New Drug is primarily indicated for the treatment of non-small cell lung cancer (ALK+) and non-small cell lung cancer (ROS1+), etc.The indication under this application is for the treatment of patients with anaplastic lymphoma kinase (ALK)-positive locally advanced or metastatic non-small cell lung cancer (NSCLC).
Foritinib is a highly potent, CNS-penetrant ALK/ROS1 inhibitor under development. The REMARK study up-to-date have demonstrated the robust efficacy in ALK-positive advanced NSCLC patients, particularly in controlling central nervous system (CNS) metastases, with a favorable safety profile. Additionally, the New Drug has completed Phase II clinical trials in mainland China for ROS1-positive NSCLC.
During the 2024 World Conference on Lung Cancer (WCLC), the interim analysis results of the Phase III REMARK study of foritinib in treating with ALK+ non-small cell lung cancer were presented as a late-breaking abstract (LBA). In this study, the foritinib treatment group demonstrated a 77% reduction in the risk of disease progression or death compared to crizotinib group (PFS NR vs 13.93m, HR 0.23, 95% CI 0.14-0.38), indicating that foritinib is a highly promising innovative drug for the treatment of ALK+ non-small cell lung cancer.
Study Methods
The REMARK study is an open-label, randomized, Phase III clinical trial that enrolled previously untreated patients with ALK-positive advanced NSCLC. Patients were stratified based on baseline ECOG performance status (0/1 vs 2) and the presence of CNS metastases, and were randomized in a 1:1 ratio to receive either foritinib (160 mg once daily) or crizotinib (250 mg twice daily). The primary endpoint was PFS assessed by an Independent Review Committee (IRC), with secondary endpoints including PFS and Objective Response Rate (ORR) assessed by investigators (INV); Time to Response (TTR) and Duration of Response (DOR) assessed by both IRC and INV; intracranial ORR (C-ORR), intracranial TTR (C-TTR), intracranial DOR (C-DOR), and time to CNS progression (CNS-TTP) assessed by IRC; as well as Overall Survival (OS) and safety.
Study Results
From December 2020 to March 2022, a total of 275 patients were enrolled in the study, with 139 receiving foritinib treatment and 136 receiving crizotinib treatment. As of March 2024, with a median follow-up of 16.7 months, the study found that the foritinib treatment group had a significantly improved PFS compared to the crizotinib treatment group. The median PFS for the crizotinib group was 13.93 months, while the foritinib group had not reached median PFS (HR 0.23, 95% CI 0.14-0.38, Figure 1).
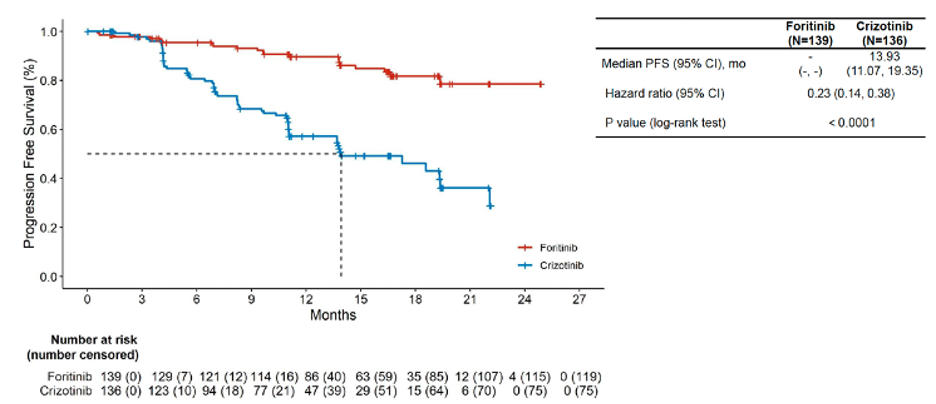
Figure 1. IRC-assessed PFS in the Intention-To-Treat population
Additionally, foritinib significantly reduced the risk of CNS progression compared to crizotinib. The median CNS-TTP for the crizotinib group was 19.32 months, whereas the foritinib group had not reached median CNS-TTP (HR 0.04, 95% CI 0.01-0.14). Furthermore, there was a trend towards improved OS with foritinib (HR 0.60, 95% CI 0.30-1.20).
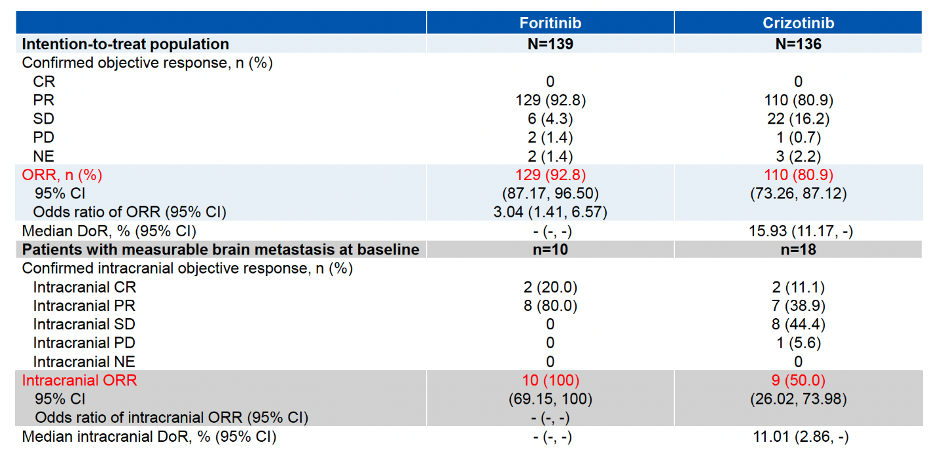
The ORR in the foritinib treatment group reached 92.8%, an increase of 12% compared to the crizotinib group (P=0.0035). For patients with baseline brain metastases, the intracranial ORR was 100% in the foritinib group compared to 50% in the crizotinib group (Table 1).
Table 1. Objective Response of Either Treatment Group
In terms of safety, the incidence of Grade 3/4 treatment-related adverse events (TRAEs) was 37.7% in the foritinib group and 55.6% in the crizotinib group. The most common Grade 3/4 TRAEs in the foritinib group were hyperglycemia, hypertension, and QT interval prolongation. No cases of interstitial lung disease or visual impairmentwere observed in the foritinib group. No fatal TRAEs were reported in either group (Table 2 & Figure 2).
Table 2. Adverse Events of Either Treatment Group
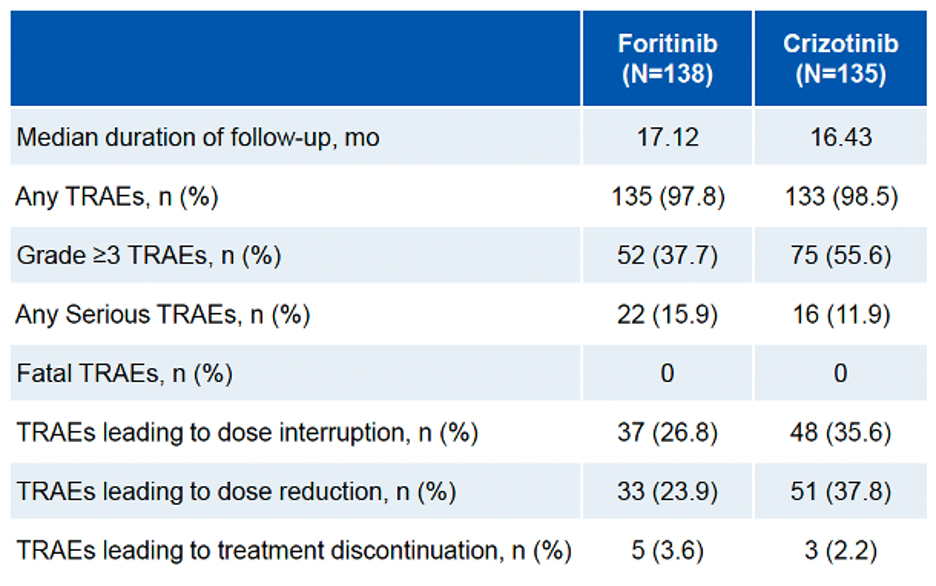
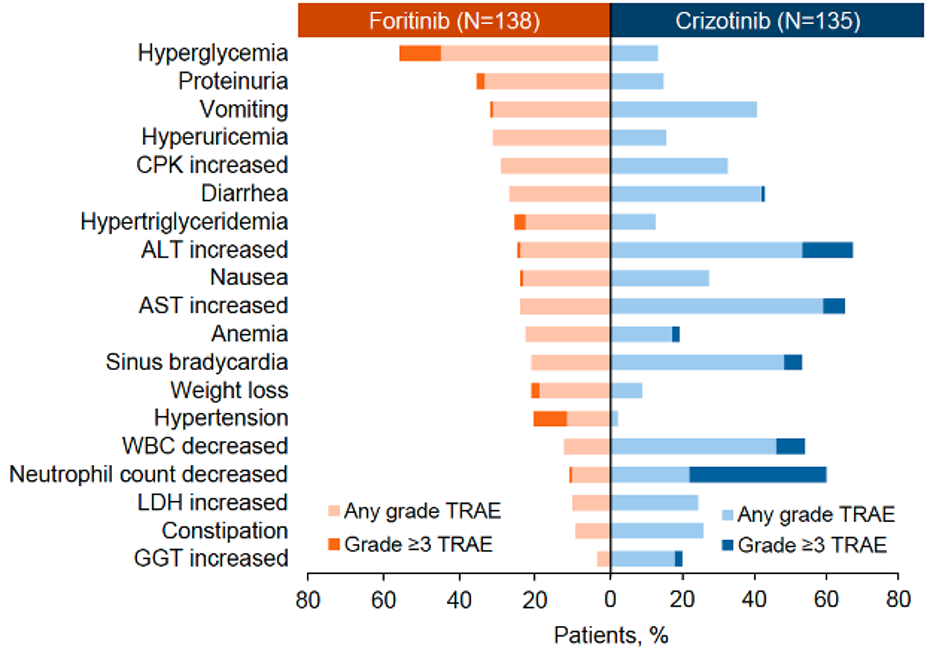
Figure 2. TRAEs Occurring in ≥20% of Patients
In summary, the study results indicate that foritinib has good overall efficacy, significantly improves PFS compared to crizotinib, reduces the risk of CNS progression, and has a controllable safety profile with no new safety signals observed post-treatment. The emergence of foritinib is expected to overcome current clinical challenges in the treatment of ALK-positive NSCLC and provide new therapeutic options for NSCLC patients.
***
Reference:
1. Xiong A. Randomized, Open-label, Phase III Study of SAF-189s Versus Crizotinib in First-Line ALK-Positive Advanced Non-Small Cell Lung Cancer (NSCLC). WCLC 2024.
2. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229-263. doi:10.3322/caac.21834
3. Han B, Zheng R, Zeng H, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent. 2024;4(1):47-53. Published 2024 Feb 2. doi:10.1016/j.jncc.2024.01.006.
4. Bareschino MA, Schettino C, Rossi A, et al. Treatment of advanced non small cell lung cancer. J Thorac Dis. 2011;3(2):122-133. doi:10.3978/j.issn.2072-1439.2010.12.08.
5. Gou LY, Wu YL. Prevalence of driver mutations in non-small-cell lung cancers in the People's Republic of China. Lung Cancer (Auckl). 2014;5:1-9. Published 2014 Feb 12. doi:10.2147/LCTT.S40817.
6. Yang JJ, Zhou J, Liu SM, et al. foritinib in advanced ROS1-rearranged non-small-cell lung cancer in China: a multicentre, open-label, single-arm, phase 2 study. Lancet Respir Med. 2024;12(9):671-680. doi:10.1016/S2213-2600(24)00171-1.

