Easier, Faster and Safer! Fosun Pharma's Self-developed Argesun® Brings a New Era in Severe Malaria Treatment
Malaria was once one of the long-lasting, widely spread, and life threatening infectious diseases in China. The country once reported 30 million cases of malaria annually in the 1940s. On 30 June 2021, the World Health Organization (WHO) officially awarded a malaria-free certification to China. However, malaria remains one of the biggest public health challenges in 84 countries worldwide, particularly in Africa. In recent years, China has been actively supporting African countries in malaria prevention and control and other health topics, contributing its knowledge and experience to help consolidate the global public health system and build a global community of health for all.
On 12 October 2023, the academic symposium "Treating Severe Malaria in a New Era", co-hosted by the CECM Medical Services, Public Health and Sanitation, Kisumu and Fosun Pharma was successfully held in Kisumu, Kenya. It was the first high-standard offline malaria academic seminar sponsored by Fosun Pharma in Africa after the COVID-19 global pandemic. Dr. Kibor Keitany, the program manager of Kenya National Malaria Control Program, Professor Arjen Dondorp from the MORU Tropical Health Network and University of Oxford, Professor Gilbert Onyango Kokwaro from Strathmore University Kenya, Prof. Robert Opoka from Aga Khan University Medical College Kenya and Dr. Walter Otieno from Maseno University School of Medicine Kenya delivered keynote speeches and scientific presentations about severe malaria management. They shared the trends in severe malaria incidence and malaria deaths worldwide, particularly in Africa in the last two decades, as well as the latest severe malaria clinical management strategies with over 30 malaria technical officials and academic experts from Kenya and its neighboring countries including Uganda, Tanzania, South Sudan, and Zambia, as well as 120 frontline healthcare workers from Kisumu, Kenya. In addition, the sumposium also had more than 50 malaria prevention and control experts and clinicians from six English-speaking African countries with high malaria prevalence, including Malawi and Ghana, joined online.

Professor Arjen Dondorp from MORU & University of Oxford delivered a keynote speech titled "How many lives can be saved with injectable artesunate?", in which he stated, "Injectable artesunate is currently still the most effective drug for the treatment of severe malaria caused by Plasmodium falciparum. Extrapolating the more than half a million malaria attributable annual deaths in Sub-Saharan Africa reported by the WHO World Malaria Report, injectable artesunate could save an additional 150,000 African children each year compared to quinine, provided all children with severe malaria can be reached for treatment. The new formulation of injectable artesunate with a single solvent system is more convenient to use and likely less prone to error. This could improve clinical practice, benefiting critically ill children with severe malaria in remote areas of Africa."
Between 2003 and 2010, Professor Arjen Dondorp led the two largest randomized clinical trials in severe malaria, SEAQUAMAT and AQUAMAT, which showed the superiority of artesunate over quinine to save lives in patients with severe malaria and changed the WHO recommended first-line treatment of severe malaria for both adults and children from quinine to injectable artesunate in the second edition of the Guidelines for the Treatment of Malariain 2011. Since 2011, the deployment of injectable artesunate in Africa has helped effectively reduce malaria mortality among African children.
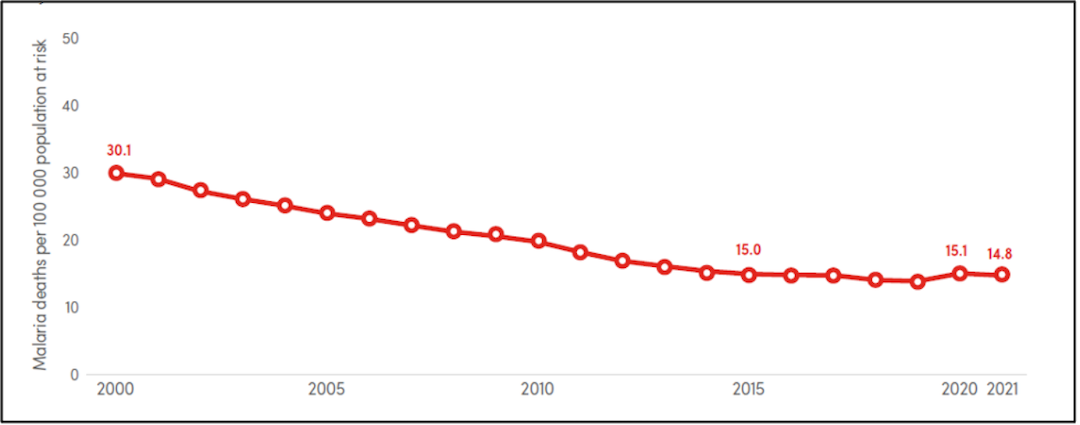
Global trends in malaria mortality rate (deaths per 100 000 population at risk), 2000–2021 (Data source: WHO World Malaria Report 2022)
The latest World Malaria Report released by the WHO in December 2022 estimated 619,000 malaria deaths globally in 2021. WHO African Region carries the heaviest malaria burden, with an estimated 234 million cases (95%) and 593,000 deaths (96%) in 2021. Nearly 80% of malaria deaths in the African region occur among children under five years old.
Kenya is one of the high malaria burden countries, with approximately 6.7 million malaria cases in 2021. The western region around Lake Victoria, with Kisumu as a center, and the eastern coastal region centered around Mombasa are the two major malaria-endemic areas in Kenya. Kisumu is also the malaria academic research center of Kenya and the entire East Africa. The Kenya National Malaria Research Institute and the malaria research sites of several international academic institutions are based in Kisumu.
The artemisinin based antimalaria medicines originated from China have made significant contributions to global malaria prevention and control since 2000. In Sub-Saharan Africa alone, about 240 million people benefit from artemisinin-based combination therapies every year. Artesun® (artesunate for injection) innovated and self-developed by Fosun Pharma is the preferred treatment of severe malaria recommended by the WHO. It has become a well-known Chinese innovator drug in Africa and the world. So far, Artesun® has been used to treat more than 60 million patients with severe malaria worldwide, most of whom were African children under five years old.
In his academic report Post-discharge Malaria and SMART Discharge, Professor Robert Opoka from Aga Khan University Medical College Kenya pointed out that the clinical management of severe malaria patients has evolved from the standard complete treatment approach of "parenteral treatment with injectable artesunate during the critical phase and one course of oral aratemisinin-based combination therapy (ACT) after discharge" to a new concept called "post-discharge malaria chemoprevention (PDMC)" for critically ill children with severe malaria and severe anemia. Studies proved that PDMC with a continuous three-month follow-up course of oral ACT can prevent the patient from a second malaria infection in such period. The new PDMC approach can effectively reduce the risk of malaria-related deaths and re-hospitalization of severe malaria children with anemia who have been discharged. Furthermore, PDMC helps in reducing the occurrence of related diseases such as anemia, severe malaria, and uncomplicated malaria in this group. PDMC was recommended by the WHO in its latest WHO Guidelines for Malariain 2022.

In June 2023, the second-generation artesunate for injection Argesun® independently developed by Fosun Pharma was prequalified by the WHO (WHO-PQ), becoming the first injectable artesunate presented with a single solvent system with WHO prequalification. It is expected to enhance the accessibility of high-quality innovative antimalarial drugs and to improve severe malaria management in primary medical institutions, further saving more lives. Up to now, Argesun® has been registered in 18 African countries.
Compared with the conventional injectable artesunate, the new generation Argesun® has simplified the preparation process of artesunate solution for injection by combining the two reconstitution and dilution steps into a single step. This improvement has significantly shorten the preparation time from 3 minutes to 1 minute, making the clinical use much easier and faster. The new Argesun® is particularly practicable for remote areas in Africa.
Effectively reduce the incidence rate of severe malaria in children from treatment to prevention
As an innovation-driven global pharmaceutical and healthcare industry group, Fosun Pharma has independently developed innovative antimalarial products covering malaria prevention and treatment, as well as the rescue of critically ill patients. From treatment to prevention, Fosun Pharma continues to provide more prevention and treatment solutions for malaria patients, especially children.
In August 2018, Fosun Pharma's SPAQ-CO® Disp (co-packed sulfadoxine-pyrimethamine dispersible tablets and amodiaquine dispersible tablets), for the prevention of malaria in children, was prequalified by WHO. Fosun Pharma has actively carried out community-oriented "Child Malaria Prevention Knowledge Popularization Projects" in 14 malaria-endemic countries in Africa. These projects aim to enhance local awareness of malaria prevention among African populations, thus helping to reduce the incidence of malaria and interrupt community transmission of the disease. By the end of June 2023, more than 245 million children in Africa had benefited from the "Seasonal Malaria Chemoprevention Program", for which SPAQ-CO® Disp is used as the core drug, effectively reducing the incidence of malaria in children under five years old in Africa.
Continue to improve the accessibility and affordability of medical and health products from "Use in Africa" to "Made in Africa and Use in Africa"
From "Use in Africa" to "Made in Africa and Use in Africa", Fosun Pharma has comprehensively improved the accessibility and affordability of medical and health products in Africa. In October 2021, Tridem Pharma Côte d'Ivoire, a subsidiary of Fosun Pharma, opened in Abidjan, the economic capital of Côte d'Ivoire in West Africa, marking the official operation of Tridem Pharma's first regional pharmaceutical distribution hub in Africa. Tridem Pharma's pharmaceutical distribution hub in Côte d'Ivoire is the first specialized logistics and distribution platform for medical and health products in French-speaking Sub-Saharan Africa, with a total storage capacity of 12,000 pallets, ensuring the sustainable accessibility of medical and health products in Africa.
In November 2022, Fosun Pharma laid the foundation stone for its park in Côte d'Ivoire, marking an important step for Fosun Pharma's strategy to achieve "Made in Africa and Use in Africa". The planned Fosun Pharma's park in Côte d'Ivoire is located in Grand Bassam. The project is planned to be carried out in three phases. After the completion of the whole project, the production capacity of the park is expected to reach 5 billion tablets per year and there will be a warehouse with a storage capacity of 10,000 pallets. The project is expected to create nearly 1,000 jobs in Grand Bassam, effectively promote the development of the pharmaceutical industry in Côte d'Ivoire, and help enhance the country's influence in the medical and health sector in West Africa.

