Fosun Pharma Receives WHO Prequalification for Its Second-generation Artesunate for Injection Argesun®
The second-generation artesunate for injection Argesun® independently developed by Fosun Pharma (600196.SH; 02196.HK) was approved by WHO Prequalification(1)(WHO PQ) on 27 June 2023, which can enhance the accessibility of innovative antimalarial drugs and ultimately contribute to saving more lives.
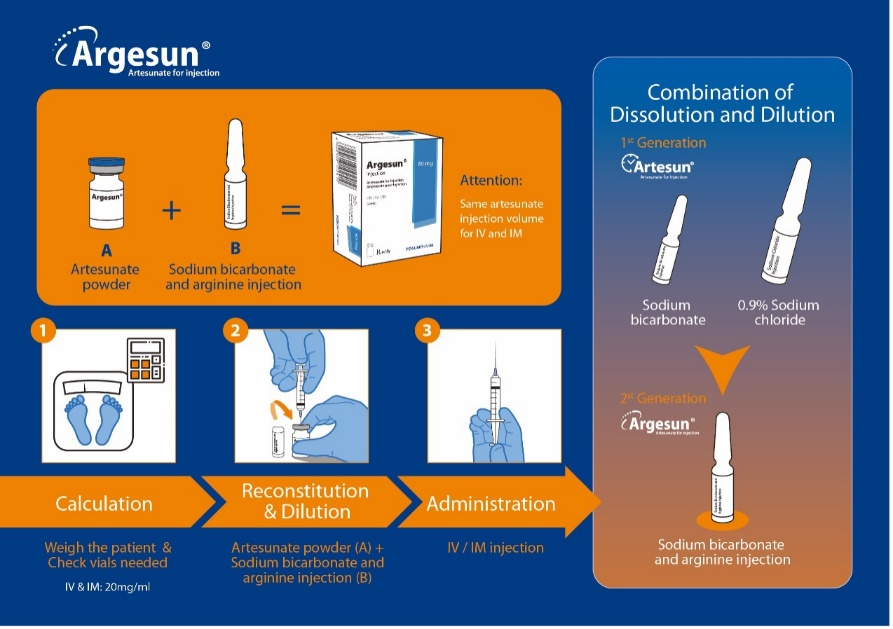
The first generation artesunate for injection (brand name Artesun®), independently developed by Fosun Pharma was approved by WHO PQ on 5 November 2010. At present, it has been successfully commercialized in 37 countries. On 27 June 2023, the second generation artesunate for injection (brand name Argesun®) became the first artesunate injectable presented with a single solvent system approved by WHO PQ and has already been registered in 16 countries.
Self-developed innovator drug artesunate for injection as a globally recognized gold standard treatment for severe malaria
The latest WHO’s World Malaria Report estimated 619,000 malaria deaths globally in 2021.WHO African Region carries the heaviest malaria burden, with an estimated 234 million cases (95%) and 593,000 deaths (96%) in 2021. Nearly 80% of malaria deaths in the African region occur among children aged under five years.
In 1987, artesunate, developed independently by Guilin Pharma, amember company ofFosun Pharma, was authorized the Class 1 New Drug Certificate No. x-01 from the dissolved Ministry of Health of the People’s Republic of China. At the same time, artesunate for injection was authorized the new drug certificate No. x-02. The clinical deployment of artesunate had played a crucial role in saving the lives of severe malaria patients, therefore WHO listed artesunate as one of the essential antimalaria medicines in 2000.
The AQUAMAT study, published in The Lancetin 2010, reported that the mortality rate was 8.5% (230 out of 2,712) for patients treated with artesunate compared to a higher rate of 10.9% (297 out of 2,713) for patients treated with quinine(2). In early 2011, the WHO updated its Guidelines for the Treatment of Malaria, recommending replacing quinine with injectable artesunate as the preferred treatment for severe malaria in all age groups. By the end of 2022, Fosun Pharma had supplied over 280 million vials (60mg) of the first-generation artesunate for injection, treating more than 56 million severe malaria patients worldwide. Fosun Pharma’s achievement stands as a flagship of China-developed innovator drugs internationally recognized.

Artesunate for injection has established itself as the globally recognized gold standard treatment of severe malaria, significantly contributing to the decline in malaria-related deaths in Africa. The deployment of injectable artesunate is estimated to safe nearly 100,000 additional lives annually in Africa alone, primarily among children aged under five years. The launch of the new generation artesunate for injection (Argesun®) is expected to improve severe malaria management in primary medical institutions, further saving more lives.
Fosun Pharma’s self-developed innovative antimalarial products portfolio covers malaria prevention, and treatments of complicated malaria and severe malaria. Currently, Fosun Pharma has 31 WHO prequalified antimalarial products, which positions it a leading supplier of antimalarial medicines worldwide. Fosun Pharma commits to provide more prevention and treatment solutions for malaria patients, especially children. By the end of 2022 there were estimated 210 million children in high malaria transmission Africa countries had been benefited from the "Seasonal Malaria Chemoprevention Program, SMC", for which SPAQ-CO® Disp is used as the core drug. with SPAQ-CO® Disp effectively reduced the morbidity and mortality of malaria in children under five years old in Africa. Looking ahead, Fosun Pharma’s global operational networks will further improve the availability and affordability of our antimalarial products so as to contribute Chinese wisdom for a Malaria-free world.
1WHO: First prequalified artesunate injectable presented with a single solvent system
https://extranet.who.int/pqweb/news/first-artesunate-injectable-single-solvent-system
2The Lancet, Volume 376, Issue 9753, Pages 1647 - 1657, 13 November 2010

