Fosun Pharma Announces Results for the First Three Quarters of 2022
Release time:2022-10-30
Content sourced from:
Page View:
Stable Growth in Revenue and Recurring Income,
Achieving a Year-on-year Increase of the Net Profit (After Extraordinary Gain or Loss) Attributable to Shareholders of the Listed Company of 15.51%, and Promoting the Internationalization Strategy Consistently
(October 30, 2022, Shanghai, China) On October 30, 2022,Shanghai Fosun Pharma (Group) Co., Ltd.("Fosun Pharma" or "the Group", Stock Code: 600196.SH; 02196.HK), a global innovation-driven pharmaceutical and healthcare industry group deep-rooted in China, announced its results for the first three quarters of 2022.
In the first three quarters of 2022, Fosun Pharma achieved steady growth in operating revenue and recurring income, of which the operating revenue was RMB 31,610 million, with a year-on-year (YOY) increase of 16.87%; net profit (after extraordinary gain or loss) attributable to shareholders of the listed company amounted to RMB 2,859 million, with a YOY increase of 15.51%;andnet cash flow from operating activities amounted to RMB 3,174 million, with a YOY increase of 5.24%.
Consistently Increasing the R&D Investment and Promoting the R&D and Registration of Innovative Products
Taking innovation as the core driver, Fosun Pharma accelerates the development and conversion of innovative technologies and products via diverse and multi-level cooperation modes, includingindependent R&D, co-development, in-licensing, and in-depth incubation.In the first three quarters of 2022, Fosun Pharma has consistently strengthened its investment in R&D, with a total R&D expenditure of RMB 3,761 million, a YOY increase of 19.36%,including R&D expenses of RMB 2,849 million, a YOY increase of RMB 435 million or a YOY increase of 18.02%.
In the first three quarters of 2022, Fosun Pharma consistently promoted the R&D and registration of innovative products and continuously launched innovative products. The application for marketing registration of the CAR-T cell therapeutic product Yi Kai Da (Ejilunsai injection) third indication (for the treatment of adult large B-cell lymphoma (r/r LBCL), which does not respond to first-line immunochemotherapy or relapses within 12 months after first-line immunochemotherapy) from Fosun Kite, a joint venture, was accept, and has been granted with priority review in October 2022.
In terms of biopharmaceuticals, Fosun Pharma has actively promoted the application for new indications of Han Si Zhuang (Serplulimab Injection) independently developed by Fosun Pharma since this product was approved for marketing in March 2022. As of October 30, 2022, the marketing applications for a total of three indications of Han Si Zhuang were accepted by the National Medical Products Administration. In addition, Han Qu You (Trastuzumab Injection) independently developed by Fosun Pharma and licensed to Cipla Limited and its holding subsidiaries was approved for marketing in Australia in July 2022. In Australia, the indications approved for the product have covered all the approved indications for the brand-name medicines.
In the first three quarters of 2022, Fosun Pharma has consistently promoted the vaccination of mRNA COVID-19 vaccine Comirnaty and accelerated the registration of new dosage forms in Hong Kong SAR, Macau SAR and Taiwan region. Formulations of BNT162b2 for infants aged 6 months to 4 years old and for children aged 5 to 11 years old have been approved in Hong Kong SAR, Macau SAR and Taiwan region, continuously strengthening the protection for young children. In addition, Omicron BA.4/BA.5-adapted bivalent COVID-19 vaccine was approved for emergency use in Taiwan region, and the applications for emergency use and special import were submitted in Hong Kong SAR and Macau SAR respectively, strengthening the local immune barrier against COVID-19.
Commercialization of Azvudine to Fight Against COVID-19 in China
In July 2022, Fosun Pharma and Genuine Biotech entered into an agreement in relation to the strategic collaboration on the joint development and Fosun Pharmaceutical Industrial’s exclusive commercialization of Azvudine. The cooperation scope includes the treatment and prevention of Novel Coronavirus (2019-nCoV) and AIDS. The Azvudine tablet is the first domestic small molecular oral drug for COVID-19 antiviral approved for launching in China, and is included in the Diagnosis and Treatment Protocol for COVID-19 (Version 9)in August 2022
Up to now, Azvudine Tablet has been listed on the procurement platform of medical insurance system in 31 provinces, autonomous regions and municipalities of China, with a price of RMB 270/bottle (35 tablets/bottle, 1 mg/tablet).
In order to improve the terminal accessibility of Azvudine Tablets, Fosun Pharma has signed a Strategic Partnership Agreement with Sinopharm Group, a leading pharmaceutical distribution enterprise in China, to accelerate the coverage of the national channel network. Up to now, Azvudine Tablet has been supplied to Xinjiang, Hainan, Henan, Yunnan, Inner Mongolia, etc., to fight against COVID-19.
Global Collaboration to Enhance Global Competitiveness
While consistently strengthening its independent innovative R&D, Fosun Pharma has also continuously deepened external collaboration, enriched the pipelines of innovative products and strengthened the global operation. Relying on the industry-leading two-way licensing capability and global operation advantages, Fosun Pharma maximizes the value of the innovative products with collaboration partners.
In September 2022, Fosun Pharma and Neovii Pharmaceuticals AG, Switzerland, entered into an exclusive agreement, under which Neovii has granted Fosun Pharma Industry an exclusive right to develop and commercialize Neovii's Grafalon®(rabbit anti-human T-lymphocyte globulin) in China.In September 2022, Fosun Pharma and Jiangsu Carephar Pharmaceutical Co., Ltd. entered into an agreement on the joint development and Fosun Pharmaceutical Industrial’s exclusive commercialization of Keverprazan Hydrochloride worldwide. The collaboration scope includes all indications of Keverprazan Hydrochloride in oral drug and injection formulations.
In addition, in October 2022, Fosun Pharma and China Resources Pharmaceutical entered into a Strategic Partnership. The two parties will focus on the field of innovative drugs, biological drugs, medical devices and other comprehensive health products to strengthen global operation and industrialization development in relevant field through strategic and business collaboration.
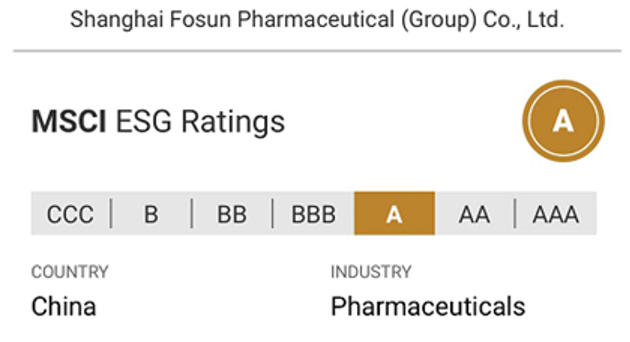
Upgrading the ESG Rating to A and Actively Implementing Global Governance Practice
Fosun Pharma has continuously improved the ESG system, promoting its long-term sustainable development. With its excellent ESG performance, Fosun Pharma was listed in the Top 40 of 2022 Fortune China ESG Impact List.The MSCI ESG rating for Fosun Pharma was further upgraded from BBB to A in October 2022, leading the industry in China.
About Fosun Pharma
Founded in 1994, Shanghai Fosun Pharmaceutical (Group) Co., Ltd.("Fosun Pharma"; stock code: 600196. SH, 02196. HK) is a global innovation-driven pharmaceutical and healthcare industry group deep-rooted in China. Fosun Pharma directly operates businesses including pharmaceutical manufacturing, medical devices, medical diagnosis, and healthcare services. As a shareholder of Sinopharm Co., Ltd., Fosun Pharma expands its areas in the pharmaceutical distribution and retail business.
Fosun Pharma is patient-centered and clinical needs-oriented. The company enriches its innovative product pipeline through diversified and multi-level cooperation models such as independent research and development, cooperative development, license-in, and in-depth incubation. Fosun Pharma has formed technological platforms for innovative small molecule drugs, antibody drugs, and cell therapy with a focus on key disease areas including oncology and immunomodulation, metabolism and digestive system, as well ascentral nervous system. Fosun Pharma also vigorously explores cutting-edge technologies, such as RNA, gene therapy, ADC and PROTAC, to enhance its innovation capabilities.
Guided by the 4IN strategy (Innovation, Internationalization, Intelligentization, and Integration), Fosun Pharma will uphold the development model of “innovation transformation, integrated operation, and steady growth", with the mission of creating shareholder values through strengthening its independent R&D and external cooperation and enriching its product pipelines, as well as promoting the global networks and enhancing operational efficiency. Fosun Pharma will actively promote the digital and physical business layout in the pharmaceutical and healthcare industry and is committed to becoming a first-class enterprise in the global medical and health market.
For more information, please visit our official website:www.fosunpharma.com.
Fosun Pharma Forward-looking statements
This press release contains forward-looking statements. All statements other than statements of historical fact contained in this press release, including, without limitation, the discussions of Fosun Pharma’s business strategies and expectations concerning future operations, margins, profitability, liquidity and capital resources, the future development of Fosun Pharma’s industry and the future development of the general economy of Fosun Pharma’s key markets and any statements preceded by, followed by or that include words and expressions such as “expect”, “seek”, “believe”, “plan”, “intend”, “estimate”, “project”, “anticipate”, “may”, “will”, “would” and “could” or similar words or statements, as they relate to Fosun Pharma’s or its management, are intended to identify forward-looking statements. These statements are subject to certain known and unknown risks, uncertainties and assumptions, which may cause Fosun Pharma’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by these forward-looking statements. Accordingly, you should not place undue reliance on any forward-looking information. Subject to the requirements of applicable laws, rules and regulations, Fosun Pharma does not have any and undertakes no obligation to update or otherwise revise the forward- looking statements in this press release, whether as a result of new information, future events or developments or otherwise. In this press release, statements of or references to Fosun Pharma’s intentions are made as of the date of this press release. Any such intentions may change in light of future developments. All forward-looking statements contained in this press release are qualified by reference to the cautionary statements set out above.
share

